How protein-based COVID vaccines could change the pandemic
Pamela Sherry is eager to become immunized against COVID-19. But she has put off getting a jab.
“I believe vaccines work,” she says. “I want the protection.” Yet she is prone to acute immune reactions and has blood circulation problems, so she has concerns about the shots available in the United States, where she lives — those based on messenger RNA (mRNA) and viral-vector technologies. Although safe for most of the population, they have been linked to rare but potentially severe side effects, including heart inflammation and blood clots.
Mix-and-match COVID vaccines ace the effectiveness test
So Sherry has been waiting for the menu of vaccine options available to her to expand. In particular, she is holding out for a vaccine built from purified proteins. Unlike the relatively new technologies that the mRNA and viral-vector COVID-19 shots are based on, protein vaccines have been used for decades to protect people from hepatitis, shingles and other viral infections. To elicit a protective immune response, these shots deliver proteins, along with immunity-stimulating adjuvants, directly to a person’s cells, rather than a fragment of genetic code that the cells must read to synthesize the proteins themselves.
Although protein vaccines are not yet in widespread use for COVID-19, late-stage clinical-trial data so far look promising, demonstrating strong protection with fewer side effects than other COVID-19 shots typically cause.
If such a shot were available, “I would go and get it right away,” says Sherry, who runs a stationery business out of her home in Prosper, Texas.
Sherry’s wait could soon be over. After months of quality-control setbacks and manufacturing delays, executives at biotechnology firm Novavax in Gaithersburg, Maryland, say they are poised to submit the company’s long-awaited application for their protein-based vaccine to US drug regulators before the end of the year. (On 1 November, Indonesia granted the company’s vaccine its first emergency authorization, and regulatory filings have already been made with government agencies in Australia, Canada, the United Kingdom, the European Union and elsewhere.) Meanwhile, two vaccine makers in Asia — Clover Biopharmaceuticals, based in Chengdu, China, and Biological E in Hyderabad, India — are similarly on track to file with various national authorities in the coming weeks and months.
In a few corners of the globe — Cuba, Taiwan, and elsewhere — home-grown protein shots are already playing a role in national vaccination efforts. Now, with a wave of more such products up for approval, the shots could allay the fears of vaccine hold-outs such as Sherry, serve as booster shots, and, importantly, help to fill a void in the global pandemic response.
So far, fewer than 6% of people in low-income countries have been vaccinated against COVID-19. Protein-based vaccines — with their inexpensive production protocols and logistical advantages, including stability at a broad range of temperatures — could help to narrow the immunization gap between rich and poor countries.
“The world needs these protein-based vaccines to reach those vulnerable populations,” says Nick Jackson, head of programmes and innovative technologies at the Coalition for Epidemic Preparedness Innovations, which has invested more than US$1 billion in five protein-based COVID-19 vaccines in active development. The lion’s share is going to products made by Clover, Novavax and SK bioscience in Seongnam, South Korea. “Protein vaccines are going to beckon in a new era of COVID-19 immunization,” Jackson says.
Intrinsically slow
From the earliest days of the pandemic response, researchers anticipated that protein-based designs would be slower off the blocks than other vaccine technologies.
Companies know how to manufacture gobs of purified protein at scale — using genetically engineered cells from mammals, insects or microbes — but the process involves many steps, each of which has to be optimized for making a specific protein. “There’s an intrinsic slowness,” says Christian Mandl, a former industry executive who now consults on vaccine-development issues. Most of the protein-based vaccines currently in testing have been crafted around some version of the coronavirus SARS-CoV-2’s spike protein, which helps the virus to enter cells (see ‘Protein vaccines 101’).
COVID vaccine boosters: the most important questions
Aside from the expected delays, however, vaccine manufacturers made some avoidable errors. When drug giants Sanofi and GlaxoSmithKline (GSK) teamed up on a protein vaccine project, for example, onlookers expected clinical development to move with great haste. But the companies initially relied on faulty reagents to characterize their product, resulting in a dosing miscalculation. Early trial participants received doses that were approximately one-fifth of the planned dose.
The mistake cost Sanofi and GSK around five months in their development timeline, because they had to repeat an exploratory study to find the optimal dose for late-stage testing. Their protein-based jab is now in a phase III trial that kicked off in late May, which involves thousands of participants in Africa, Asia and Latin America.
By comparison, large-scale trials from Novavax and Clover have already yielded efficacy data. According to a preprint published last month (that has not been peer reviewed)1, the Novavax jab offered more than 90% protection against symptomatic COVID-19 in a 30,000-person study completed early in the year — before the Delta variant arrived, when only milder forms of the virus were in circulation.
Ones to watch: Vaccine makers with protein jabs in late-stage clinical trials
Company | Location | Vaccine type | Cell manufacturing system |
|---|---|---|---|
Biological E | Hyderabad, India | Soluble protein | Microbial cells (yeast) |
Clover Biopharmaceuticals | Chengdu, China | Soluble protein | Mammalian cells (hamster ovary) |
Medicago | Quebec City, Canada | Virus-like particle | Plant cells (tobacco-like Nicotiana benthamiana) |
Novavax | Gaithersburg, Maryland | Protein nanoparticle | Insect cells (fall armyworm) |
Sanofi/GlaxoSmithKline | Paris/Brentford, UK | Soluble protein | Insect cells (fall armyworm) |
SK bioscience | Seongnam, South Korea | Protein nanoparticle | Mammalian cells (human) |
Clover reported somewhat lower efficacy results for its protein-based jab — just 67% for symptomatic COVID-19 of any severity — but that number was probably deflated, because the vaccine was tested on populations grappling with more virulent strains of SARS-CoV-2, including the Delta and Mu variants. Both vaccines elicited antibody levels on par with those induced by mRNA shots, which have emerged as some of the most efficacious in the pandemic2,3.
The results show that making COVID-19 vaccines using proteins “is not a substandard approach just because it took longer,” says Ryan Spencer, chief executive of Dynavax Technologies of Emeryville, California, which makes the Clover vaccine’s adjuvant.
The tangled history of mRNA vaccines
The shots also appear to be safe. None of the 50 or so protein-based COVID-19 vaccines now in clinical testing around the world have elicited any major side effects. Even many of the reactions typically elicited by the mRNA or viral-vector jabs — headaches, fevers, nausea and chills — have proven far less common with the protein-based alternatives. For example, less than 1% of individuals who received a protein-based shot from Taiwan’s Medigen Vaccine Biologics Corporation, in Taipei City, developed fevers in clinical studies.
“The safety profile is very much like those of influenza vaccines,” says Szu-Min Hsieh, an infectious-disease specialist at the National Taiwan University Hospital in Taipei, who published phase II trial results last month4.
“That’s going to allow a lot of people not to fret as much,” adds Cindy Gay, an infectious-disease physician at the University of North Carolina School of Medicine in Chapel Hill, who co-led testing of the Novavax vaccine.
Design differences
Even if one protein-based vaccine succeeds — both in terms of its performance and in finding a market — there’s no reason to think they all will, however.
For one thing, the form of the spike protein they deploy varies greatly from one product to the next. Some use single proteins, others triads. Some use full-length spike protein, others just a fragment. Some proteins are free-floating, others are packaged together into nanoparticles.
Why is it so hard to investigate the rare side effects of COVID vaccines?
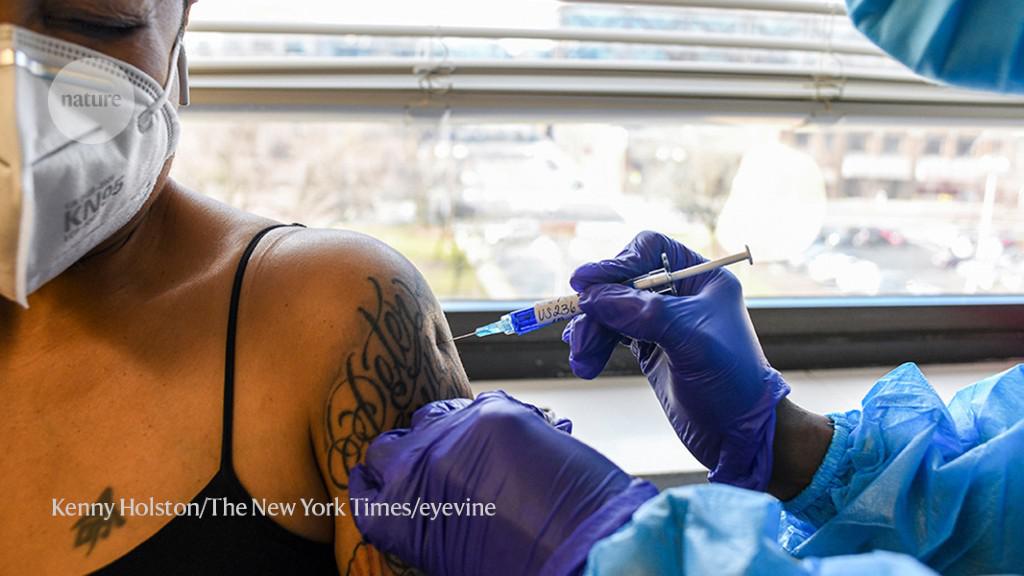
Many of them are also manufactured using different types of cell (see ‘Ones to watch’). Novavax and Sanofi/GSK use cells from the fall armyworm (Spodoptera frugiperda), a type of moth, to synthesize protein; Clover and Medigen rely on hamster ovary cells, a mainstay of therapeutic antibody production in the biotechnology industry. Plus, the leading candidates rely on different adjuvants, each of which prods the immune system in its own way, resulting in different kinds of vaccine responses.
All of this could translate into different efficacy and safety profiles, says Thomas Breuer, chief global health officer for GSK. “I could imagine that you will see differences, but time and the phase III trial results will give us the ultimate answer.”
Those results have the potential to shape booster programmes in wealthy countries, where large percentages of the population have already been vaccinated. Although mRNA jabs are currently being used as boosters in many of these places, tolerability concerns could drive people to seek out protein-based boosters once they’re available.
The technology is tried and true, and studies have shown that a mix-and-match strategy — in which a different COVID-19 vaccine is administered after the first — are effective at preventing the disease, notes John Mascola, director of the Vaccine Research Center at the US National Institute of Allergy and Infectious Diseases. “We would need to see human data” confirming such a protein-based booster regimen is similarly safe and effective, Mascola says — but he and others expect that it will be. Trials evaluating the approach are ongoing.
Plugging the equity gap
Once authorized, protein shots are also expected to rapidly address supply shortages that have plagued efforts to vaccinate lower-income countries. Novavax and Clover, for example, have each pledged to donate hundreds of millions of doses of their jabs next year to COVAX, an initiative designed to distribute vaccines around the world.
The global health community has also been arguing that equitable access to COVID-19 vaccines could be achieved through local manufacturing of shots in the global south. To achieve this, more researchers should be looking to simple, inexpensive production systems that manufacturers in less-wealthy countries can readily implement, says Christopher Love, a chemical engineer at the Massachusetts Institute of Technology in Cambridge.
The fight to manufacture COVID vaccines in lower-income countries
Biological E is already taking advantage of one such system — yeast — to manufacture the vaccine it licensed from Baylor College of Medicine in Houston, Texas. According to Maria Elena Bottazzi, a Baylor virologist who helped to create the product, that makes it “probably the easiest and cheapest to scale” of all the COVID-19 vaccines on or nearing the market today.
In the earliest days of the COVID-19 crisis, vaccine platforms such as mRNA brought the advantage of speed, says Ralf Clemens, a vaccine-industry veteran and a scientific adviser to Clover. But now that a wave of protein-based vaccines is coming, he says, they will have a lot more to offer — and in the long run when it comes to protecting the world against coronavirus infections, “I think they will prevail.”